IMBIO, a startup launched from the University of Michigan in 2007, has just received clearance from the U.S. Food and Drug Administration to sell its software platform that analyzes images of patients’ lungs with detailed precision. Called Lung Density Analysis or LDA, it allows doctors to carefully analyze a patient’s CT (computed tomography) lung scan and look at how their disease is affecting their ability to fill their lungs with air and to push it out when they exhale.
The new FDA 510(K) clearance allows doctors to use technology that may soon help lung disease patients around the world breathe a little easier, by helping their doctors make a clearer diagnosis and more individualized treatment plan.
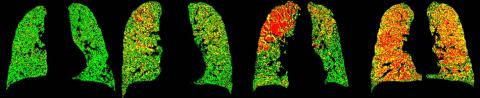
Red and yellow areas in this comparison of different patients' lungs, imaged using the U-M/Imbio technique, show reduced ability to push out air (Photo: University of Michigan Center for Molecular Imaging)
Imbio’s LDA system will particularly useful in patients with chronic obstructive pulmonary disease or COPD, which affects over 60 million people worldwide. The system uses powerful computer techniques to overlay the CT scan taken during a full inhalation with an image taken during a full exhalation. The overlaid, or registered, CT images share the same geometric space, so that the lung tissue in the inflated and deflated lungs aligns. The density of healthy lung tissue will change more between the two images than the density of diseased lung, allowing researchers to create a three-dimensional “map” of the patient’s lung function.
The software assigns colors to each small 3-D area, called a voxel, according to the difference in signal changes within each of the areas between the two scans. Green means healthy, yellow means a reduced ability to push air out of the lung’s small air sacs, and red means severely reduced ability.
Imbio co-founders, Brian Ross, Ph.D., and Alnawaz Rehemtulla, Ph.D., were behind the technology that grew out of basic laboratory research at in the University of Michigan (U-M) Department of Radiology’s Center for Molecular Imaging. Both now act as scientific advisors to Imbio.
“It’s incredibly gratifying to see this concept grow from an idea in our lab, to a product ready for market,” said Dr. Ross. “We look forward to seeing how clinicians worldwide can use the LDA approach to benefit patients, and we’re grateful to all who have helped this concept reach this exciting point through many years of research and product development.” He credited the university’s research and technology transfer environment for helping the concept reach the marketable stage.
Not only does the technology have its roots in a U-M lab, it was also tested thoroughly by a separate team of U-M Health System lung-imaging experts using images from the COPDGene trial that involved thousands of COPD patients in the United States. Together, they published the results of their in-depth testing in the journal Nature Medicine in 2012.
NIKE AIR JORDAN













