3M has unveiled a new purification technology platform designed to help biopharmaceutical manufacturers achieve high product purity early in the manufacturing process, improving efficiency and process economics.
The new 3M™ Emphaze™ AEX Hybrid Purifier, the first in a series of single-use chromatographic products, uniquely combines three 3M core technologies—advanced polymer materials, fine fiber nonwovens, and porous membranes—into a novel purification media. The result is an all-synthetic clarifying product line containing both a novel anion exchange nonwoven media and a fine particle, bioburden reduction membrane.
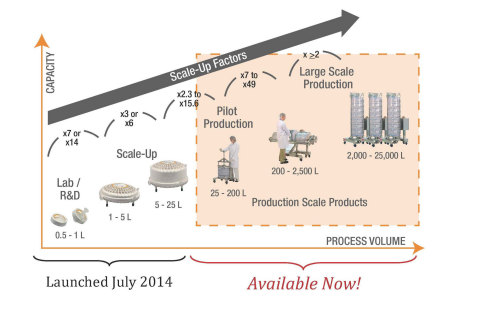
In addition to the 3M Emphaze AEX Hybrid Purifier Laboratory and Scale-Up single-use capsules introduced in July last year, the 3M Emphaze AEX Hybrid Purifier family is now complete with the introduction of two new Production Capsules.
“The 3M Emphaze AEX Hybrid Purifier is a multi-mechanism clarifying product,” explained Tom Martin, global business manager of Life Sciences Process Technologies at 3M Purification. “This actually represents a new class of clarifying products, with an all-synthetic construction, substantial anion exchange capacity, and a defined 0.2 µm pore size membrane.”
The product is an integral combination of two types of media—Q-functional nonwoven and membrane—that work together to provide unsurpassed product purity after clarification when using a single-unit operation. The unique nonwoven hydrogel together with the qualifying membrane provides tremendous removal of insoluble particles well below 0.1 µm in size, resulting in superior turbidity reduction.
At the same time, the high chromatographic capacity of the Q-functional nonwoven provides substantial reduction of negatively charged, soluble impurities including DNA and HCP. When used in mAb purification, this combination of attributes allows the hybrid purifier to provide superior product purity before the protein A affinity column, which in turn results in even greater purity downstream of the protein A operation. In turn, high-purity product enables significant process simplification in downstream processes.
“We designed the 3M Emphaze AEX Hybrid Purifier to provide the highest possible level of product protein purity early in biopharmaceutical downstream process. What is truly exciting is that by targeting the removal of particularly problematic impurities from entering the protein A column, a profound impact on the entire downstream process can be realized,” said Greg Jellum, global laboratory manager of Life Sciences Process Technologies at 3M Purification. “This new product platform is a tremendous demonstration in leveraging 3M’s materials-based technology engine to enable integrated process innovation for our industry.”
The 3M Emphaze AEX Hybrid Purifier offers a loading capacity similar to fine grades of highly charged conventional depth filters, and can easily be incorporated into existing manufacturing processes where such a depth filter would be used.
The 3M Emphaze AEX Hybrid Purifier will be encapsulated in 3M’s signature single-use capsule design. This common product design and hardware platform enables seamless integration into a 3M-matched component solution with high-capacity Zeta Plus depth filtration and LifeASSURE sterilizing grade membranes for a state-of-the-art harvest and clarification process.
By providing the best possible protection of valuable downstream unit processes, the 3M Emphaze AEX Hybrid Purifier technology holds significant potential for improving efficiency in the production of mAb drugs, other mammalian or bacterial culture produced recombinant proteins or viral vectors, or plasma fractionation processes.
Tienda outlet deportiva













