Celanese Corporation will present innovative uses of ethylene vinyl acetate (EVA) at the 2015 CPhI Worldwide conference to be held from October 13-15 in Madrid, Spain. At this event, Celanese material experts will exhibit the innovative controlled release pharmaceutical copolymer, VitalDose EVA.
EVA has been used for years in drug delivery systems, according to Dr. Donald Loveday, Celanese strategic marketing manager. VitalDose EVA is an inert thermoplastic that is expanding design possibilities with its customizable drug release properties. This controlled release excipient is developed for a wide variety of pharmaceutical products with varying routes of administration, including intraoral, intravaginal, ocular, subcutaneous and transdermal.
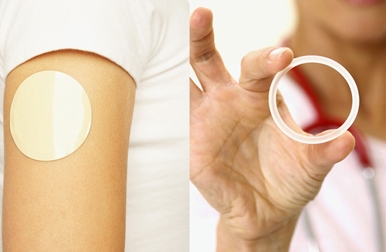
Celanese material experts will discuss topics at a Pharma Insight Briefing & Reception, such as why use EVA in drug development and controlled release; the current application areas and uses of EVA; next generation innovations for EVA; and Celanese support of conventional and novel uses of EVA.
For more information about Celanese’s controlled release pharmaceutical EVA polymer, VitalDose EVA, visit www.vitaldose.com.

 iConnectHub
iConnectHub
 Login/Register
Login/Register Supplier Login
Supplier Login



























