MILESTONE Medical, exclusive licensee of Milestone Scientific's epidural and intra-articular instruments, has entered into a distribution agreement with Milestone China.
Under the terms of agreement, Milestone China will oversee the sales and distribution of Milestone Specific’s intra-articular instruments in China and other parts of Asia. The instruments are used in the treatment of osteoarthritis.
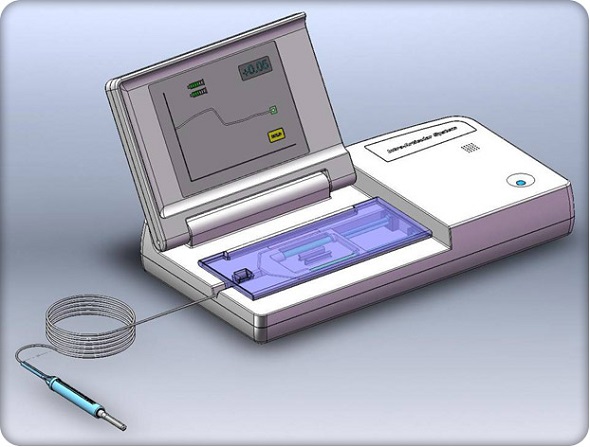
CompuFlo® offers greater ease of use and improved reliability and accuracy for osteoarthritic patients requiring intra-articular injections (Photo: Milestone Scientific)
The company has agreed to minimum annual purchases following CFDA marketing clearance as a condition of the distribution agreement. The minimum purchase for the first year is 300 instruments, which will double in year two. For year three, the minimum purchase is 800 instruments.
The CFDA marketing clearance can be sought only after obtaining FDA marketing clearance in the United States. Companies have no control over the regulatory process, but Milestone is confident it will secure approval by the end of the second quarter of 2015.
Milestone China chief executive officer Lidong Zhang said it is gratifying to add the unique precision instruments to its product portfolio to boost the treatment options and comfort level of patients suffering from osteoarthritis and other joint pains.
“Our team's experience in the Chinese marketplace combined with the benefits for both patients and medical practitioners make this a favorable agreement for all parties. We look forward to commencing commercial sales upon CFDA marketing clearance," Zhang added.
Milestone Scientific chief executive officer Leonard Osser said, "Having access to a sizable market such as China with a proven team of marketing and sales professionals is an important step forward for Milestone Scientific as we prepare our commercial launch. The fact that Milestone China has committed to guaranteed minimum purchases upon CFDA marketing clearance is further validation of our technology."
Milestone China is a joint venture between a team headed by a senior healthcare executive from China and Milestone Scientific Inc., a medical R&D company that designs, patents, incubates, and commercializes innovative injection technologies. The JV focuses on supplying and distributing medical and dental instruments, and disposables to China and other Asian markets.
Jordan Extra Fly
 iConnectHub
iConnectHub
 Login/Register
Login/Register Supplier Login
Supplier Login



























