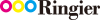Specialty materials for the medical sector have grown substantially in volume and diversity, with requirements getting more complicated and urgent. These materials, with special properties, are in high demand for medical packaging and devices, as well as for advanced medical equipment used for laboratory testing and analysis.
Celanese Corporation, a global technology and specialty materials company, has introduced Hostaform® MT® SlideX™ POM, a family of tribologically modified, medically compliant engineered materials. These thermoplastic polymers enable the production of medical devices with a very low coefficient of friction and wear, low noise (squeaking) and eliminate the need for external lubrication. Medical devices manufactured with these new materials operate smoothly with a high degree of patient comfort and consistency from the very first use.
Hostaform® MT® SlideX™ POM is a competitive alternative to various kinds of high-performance, tribologically modified compounds. When compared to alternative materials, Hostaform® MT® SlideX™ POM offers a significantly lower coefficient of friction in medical devices combined with the Celanese medical technology (MT®) service package. This results in the potential to reduce costs by removing design constraints and simplifying material combinations in complex devices while avoiding external lubrication in manufacturing processes.
The MT® service package addresses quality, change control and regulatory compliance in accordance with pharmaceutical and medical industry expectations, based on Celanese’s extensive experience with material supply to the medical market.
The advantages to the patient as a result of medical device manufacturers using Celanese’s tribologically modified Hostaform® MT® SlideX™ POM include: reduction in force required to activate the medical device; no squeaking noise when operating the medical device, and increased comfort during use due to easy sliding properties.
“The use of medically compliant polymers from Celanese is helping the medical industry to design and manufacture medical devices which can significantly increase patient comfort by reducing friction and noise,” said Andrew Brown, director of Celanese’s global medical industry platform. “These new materials can improve design and processing capability and increase performance levels of key medical components. The introduction of these low-tribological polymer grades from Celanese underscores our commitment to medical industry innovation which benefits patients who seek greater comfort and ease of use in their medical devices.”
With a high mechanical performance profile combined with the Celanese MT® service package, Hostaform® MT® SlideX™ POM is well-suited for medical applications such as COPD (Chronic Obstructive Pulmonary Disease) and asthma inhalers, injection devices such as insulin pens, surgical instruments and portable diagnostic medical devices, where low friction, wear and medical compliance are important requirements.
Patient safety as utmost objective
Eastman Chemical Company is also finding new ways to help medical device companies boost product quality and patient safety with its well-known Eastman Tritan™ copolyester. Medical adhesives developed by Dymax Corporation, a hearing screener developed by Natus Medical Incorporated and small bore connectors developed by A. Hopf GmbH are relying on the new-generation copolyester to meet customer needs and regulatory requirements while ensuring confidence in the end device.
A. Hopf uses Tritan™ copolyester for its connectors.
Dymax’s medical device adhesives, Natus Medical’s Echo-Screen® III hearing screener and Eastman’s work on small-bore connectors. These innovations reflect the adaptability of Eastman Tritan™ copolyester, which is tough, free of bisphenol A (BPA) and resistant to aggressive cleansers and disinfectants.
“Eastman Tritan™ copolyester can help improve medical device reliability and patient safety in a variety of ways — from a toughness that can translate into reduced device failure to collaborations that can ensure quality materials work well together,” said Ellen Turner, market development manager, specialty plastics, medical devices, Eastman. “Ultimately, the material provides greater peace of mind for health care professionals and patients.”
Dymax, — a leading manufacturer of advanced light-curable adhesives, coatings, oligomers, light-curing equipment and fluid dispense systems — teamed with Eastman for an efficient and dependable process to create value-added solutions. Through their joint testing, the companies found that pairing Eastman Tritan™ copolyester with select Dymax medical device adhesives provided the tough, reliable, BPA-free combination that customers need. Now, the companies have a full list of products that have been tested for biocompatibility, are supported by test data and can be recommended early in the development of new devices. The collaboration will reduce time to market for lifesaving innovations.
“Our customers value efficiency and reliability throughout the design and validation process. The sooner they can deliver safe medical devices to market, the sooner patient outcomes can be improved,” said Mark Pizzuto, global market segment manager, medical, for Dymax. “Building a bridge along the supply chain requires companies with shared values, extensive experience, and a willingness to work together. We found that Eastman valued a complete customer solution and worked with the customer needs top of mind similarly to Dymax.”
Natus Medical’s Echo-Screen® III hearing screener, made with Eastman Tritan™ copolyester MXF121, is used primarily for newborn hearing screening. This compact, all-in-one device incorporates transiently evoked otoacoustic emissions (TEOAE), distortion product otoacoustic emissions (DPOAE) and auditory brainstem response (ABR) capabilities. To answer customer needs, the global leader in newborn hearing screening solutions established new material requirements for its infant care products. The Echo-Screen III hearing screener — originally designed and tooled using polycarbonate (PC)/acrylonitrile butadiene styrene (ABS) alloy — now features Eastman Tritan™ copolyester MXF121, which is manufactured without BPA and has superior chemical resistance, along with excellent mechanical properties, impact resistance and mouldability. Tritan MXF121 also is made without halogens and ortho-phthalate plasticisers.
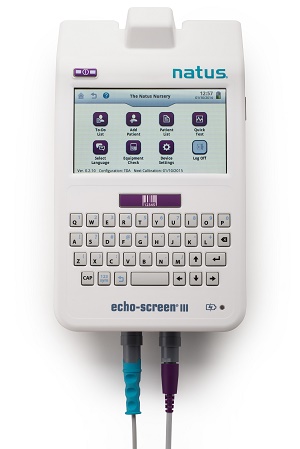
Natus Medical’s Echo-Screen® III hearing screener
Eastman Tritan™ copolyester MXF121 has helped Natus Medical proactively address patient safety concerns. “The Natus development team was able to meet user requirements for a DEHP-free and BPA-free product by partnering with Eastman on material selection,” said Jim Hawkins, president and CEO of Natus Medical.
A. Hopf GmbH selected Eastman Tritan™ copolyester as a raw material suitable for a newly designed small-bore connector. The connector addresses growing concerns for medical-device misconnections, which prompted a search for consistent, easier-to-use and safer small-bore connectors. The Global Enteral Device Supplier Association (GEDSA) already has seen regulatory changes to this effect with the International Organization for Standardization (ISO) standard for connectors for enteral applications, ISO/DIS 2 80369-3. A. Hopf GmbH proactively addressed this with a consistent design for enteral connectors according to the new requirements.
Eastman Tritan™ copolyester maintains clarity and colour after sterilisation, providing excellent aesthetic appeal and helping boost patient and health care provider confidence. In addition, devices retain clarity and functional integrity following ethylene oxide and gamma sterilisation.
Dynamic technologies for the healthcare industry
PolyOne Corporation showcased its dynamic range of technologies for the healthcare industry at Medical Design and Manufacturing (MD&M) West 2015 with healthcare material, colourant and additive solutions aimed at addressing the requirements of medical products manufacturers.
Its Versaflex™ VDT thermoplastic elastomers improve ergonomics and performance by increasing vibration damping to absorb shock. This specialty material also allows manufacturers to eliminate secondary assembly steps and streamline their processing. NEU™ View Radiopaque Translucent Solutions offer catheter producers an enhanced technology that provides excellent visibility, both optically and under X-ray, boosting clinician confidence and patient comfort. This technology eliminates the need for co-extrusion of a radiopaque stripe, reducing scrap rates and improving production efficiency.
Specialty sheet and rollstock: Valiant™ healthcare packaging solutions for sterile medical device trays and diagnostic kits include options with high barrier technology for pharmaceutical unit dosage and combination device packaging. Royalite™ flame retardant, UL- listed sheet materials provide the chemical resistance, formability and impact strength needed for large medical and electronic housings. Both Valiant and Royalite offerings meet the high quality standards and regulatory requirements of the medical industry.
Accesorios para el running
 iConnectHub
iConnectHub
 Login/Register
Login/Register Supplier Login
Supplier Login