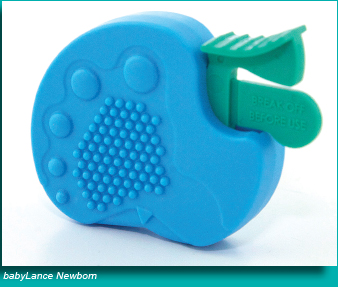

A safety medical product, the babyLance heel incision device for infants, was launched in North America and Europe by Singapore-based medical device innovator and distributor MediPurpose. The heel incision device is said to provide better control in drawing blood from newborn infants for various tests, removing the need for conventional needle pricking of fingers. Designed and invented by Sun Jian Ping, a research scientist seconded from the Singapore Institute of Manufacturing Technology (SIMTech), a research institute of the Agency for Science, Technology and Research (A*STAR), the babyLance heel incision device uses an innovative activation mechanism to improve the incision performance. It reduces the penetration depth, thus avoiding puncturing any soft bone tissue of the infant. The design of babyLance delivers incision depths that are optimal for infant heel sticks through a pendulum cutting action that creates a single perfect cut, reducing the pain during incision. Sun joined MediPurpose under A*STAR's Technology for Enterprise Capability Upgrading scheme, where A*STAR researchers are seconded to small and medium enterprises (SMEs) for up to two years to provide them with R&D capabilities to help them improve production process or develop products. The babyLance heel incision device is listed with the U.S. Food and Drug Administration and received the CE marking for Europe. The market size for baby heel incision devices in the United States is about 30 million pieces a year. "The global launch of babyLance is a testimony of SIMTech's strong ties with the industry. This collaboration between MediPurpose and SIMTech should inspire Singapore SMEs to create innovative medical devices through product design and technology. Company size is not a barrier to tap the opportunities in the growing global medtech industry," commented Dr. Lim Ser Yong, executive director of SIMTech. "We are very excited about this latest addition to our blood collection medical product portfolio," said MediPurpose founder and CEO Patrick Yi. "After successfully entering the U.S. healthcare market, we commissioned SIMTech to conduct a feasibility study of the heel incision device to complement our first product, the SurgiLance safety lancet. SIMTech followed up on that study by seconding Sun to work with our engineers." The babyLance will be available in two models: babyLance Newborn (BLN), which delivers an incision depth of 1 mm, and babyLance Preemie (BLP), which delivers an incision depth of 0.85 mm. In particular, the babyLance heel incision device includes thoughtful innovative features for end-users in providing safety, comfort and ease of use: Ease of activation: Reduces trigger activation force, thereby significantly reducing the risk of bruising Unique positioning design: Facilitates a stable and accurate placement against the targeted section of the infant's heel while ensuring the procedure can be performed consistently and quickly Compliance with regulatory and quality standards: ThebabyLance cutting blade's swift pendulum action makes an incision that complies with the CLSI LA4-A5 established guidelines Cost-effectiveness: The babyLance heel incision device incorporates unique design characteristics that allow for the most cost-effective manufacturing process. (the end) Source: MediPurposeNike Air Jordan 11Lab4 Retro 4 Patent Leather

 iConnectHub
iConnectHub
 Login/Register
Login/Register Supplier Login
Supplier Login



























