 NUTRITION and health claims on food labels can be a useful public health tool for the promotion of nutrition awareness. Such information is also beneficial to food industry, enabling manufacturers to highlight the nutritional quality of their products. Recognising this, there has been increased interest and efforts of by authorities to in improve improving regulatory control of regulations concerning nutrition labelling and health claims, to ensure that such information are is accurate and appropriate. This article provides an overview of the regulatory status of nutrition and health claims for nutrients and bioactive compounds in selected countries in the Asian region*. Definition of nutrition and health claims The following is a summary of the definition of nutrition and health claims according to Codex Alimentarius, an international food standards setting organisation. Nutrition claim means any representation, which states, suggests or implies that a food has particular nutritional properties. There are two types of nutrition claims, namely (a) nutrient content claim (e.g. high in vitamin C; free of cholesterol) and (b) comparative claim (e.g. more protein, less sodium). Health claim means any representation that states, suggests or implies that a relationship exists between a food or a constituent of that food and health. These include: (a) nutrient function claim, (b) other function claim and (c) reduction of disease risk claim.
NUTRITION and health claims on food labels can be a useful public health tool for the promotion of nutrition awareness. Such information is also beneficial to food industry, enabling manufacturers to highlight the nutritional quality of their products. Recognising this, there has been increased interest and efforts of by authorities to in improve improving regulatory control of regulations concerning nutrition labelling and health claims, to ensure that such information are is accurate and appropriate. This article provides an overview of the regulatory status of nutrition and health claims for nutrients and bioactive compounds in selected countries in the Asian region*. Definition of nutrition and health claims The following is a summary of the definition of nutrition and health claims according to Codex Alimentarius, an international food standards setting organisation. Nutrition claim means any representation, which states, suggests or implies that a food has particular nutritional properties. There are two types of nutrition claims, namely (a) nutrient content claim (e.g. high in vitamin C; free of cholesterol) and (b) comparative claim (e.g. more protein, less sodium). Health claim means any representation that states, suggests or implies that a relationship exists between a food or a constituent of that food and health. These include: (a) nutrient function claim, (b) other function claim and (c) reduction of disease risk claim. 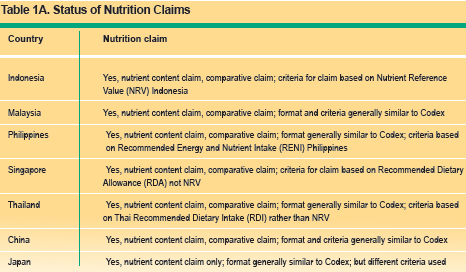
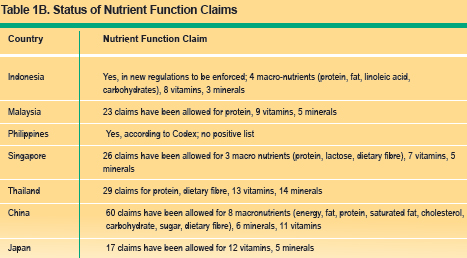
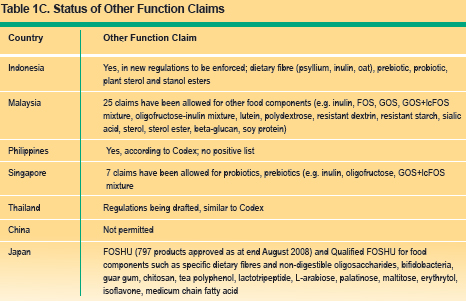
Nutrient function claims describes the physiological role of the nutrient in growth, development and normal functions of the body, e.g. calcium aids in the development of strong bones and teeth. Other function claims describes specific beneficial effects of the consumption of a food constituent (or bioactive compound) in improving or modifying a physiological function. In recent years, there has been considerable interest in role of bioactive components or functional ingredients in promoting health. Another example of such function claim is "plant sterols help in lowering blood cholesterol". Reduction of disease risk claims relates to the consumption of a food or food constituent (such as bioactives) to the reduced risk of developing a disease or health related condition. An example of such a claim is: soy protein reduces risk to of developing heart disease. Tables 1A ?1C summarise the permitted nutrition claims, nutrient function claims, other function claims and disease risk reduction claims in the countries reviewed.
 Conclusions There is continuing interest in nutrition and health claims amongst the food industry in the region. The food industry would like to have a clear regulatory framework for application of nutrition and health claims. It also seeks a reasonable lag time between application and final approval of a claim. Smaller companies also lack expertise to submit such applications. There is increasing emphasis on the need for scientific substantiation before health claims are permitted. The authorities are looking into putting in place clear regulatory systems to facilitate making of nutrition and health claims by the food industry. As different countries work internally to set up systems that work for them, there is a danger that differing regulations can cause confusion for consumers and food companies and inhibit free trade of food products from one country to another. It would seem that the need to keep consumers well informed on product benefits based on sound science should have more similarities than differences amongst countries and hopefully the Codex system will help provide guidance in that direNike Hypervenom Phantom
Conclusions There is continuing interest in nutrition and health claims amongst the food industry in the region. The food industry would like to have a clear regulatory framework for application of nutrition and health claims. It also seeks a reasonable lag time between application and final approval of a claim. Smaller companies also lack expertise to submit such applications. There is increasing emphasis on the need for scientific substantiation before health claims are permitted. The authorities are looking into putting in place clear regulatory systems to facilitate making of nutrition and health claims by the food industry. As different countries work internally to set up systems that work for them, there is a danger that differing regulations can cause confusion for consumers and food companies and inhibit free trade of food products from one country to another. It would seem that the need to keep consumers well informed on product benefits based on sound science should have more similarities than differences amongst countries and hopefully the Codex system will help provide guidance in that direNike Hypervenom Phantom
 iConnectHub
iConnectHub
 Login/Register
Login/Register Supplier Login
Supplier Login


























