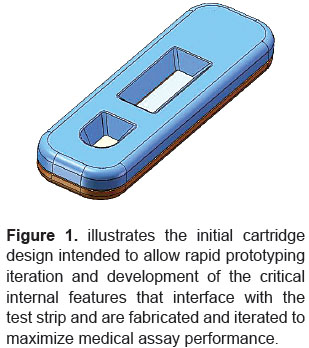
 The Marketplace Challenge: Two words best encompass a challenge often faced by medical device designers and manufacturers: "speed" and "reliability". Intense competitive pressures, combined with demands from the medical community for solutions to combat the spread of disease, have created a marketplace in which even months can be too long for companies to react and produce relevant products. And yet, speed-to-market must be achieved without compromising the critical need for medical products to perform as required in accordance with highly technical specifications, many of which are predicated upon accuracy and durability. Symbient Product Development strives to achieve both speed and reliability by blending current science and engineering theory with solid design principles for the creation of medical device and diagnostic products. In that process, our ability to evaluate a design and anticipate product performance is often highly dependent upon the quality and comprehensiveness of prototyping technology. This case study will focus on the vital role played by WaterShed?XC 11122, a rapid prototyping resin from DSM Somos, which helps to ensure a remarkably fast turnaround coupled with the ability to replicate actual performance attributes of production-grade engineered thermoplastics. The Product Challenge: Symbient Product Development was given the opportunity to participate in a fast-timeline project to develop a Diagnostic Lateral Flow Cartridge. This device uses a lateral flow test strip to evaluate, within a matter of minutes at the point-of-care, whether or not a patient is infected with a virus. In conducting such a test, a fluid sample is collected from the patient and mixed with a buffer, after which several drops of the combined sample and buffer are added to a sample well. The sample is applied, test strips run, and a clinician then places the Diagnostic Lateral Flow Cartridge into an instrument that reads the test result. Our design assignment, in parallel with the development of the internal cartridge features, was to develop an exterior industrial design that includes the user interface, features to aid in handling and insertion into the instrument, and aesthetic factors intended to give the product a unique appearance. In accepting this product design project Symbient Product Development committed to the necessity of completing our responsibilities within four months ?an extremely fast turnaround 梬ithout sacrificing the quality of prototyping evaluations that would assure the end-user that the Diagnostic Lateral Flow Cartridge would perform as required. Solving the Challenges: Our approach to meeting challenges of both the medical device marketplace and the individual product for which we were responsible, was based on a procedure almost identical to the accepted four-step plan for handling a medical emergency: Step 1: Establish a Planning Team Our first goal was to ensure that our vision and expertise aligned with our client's vision and expectations. This required input from our designers, engineers and administrators, but also the experience of professional resources such as DSM Somos, a world leader in high-performance stereolithography resins. We knew, from prior experience, that DSM's rapid prototyping materials would provide us with the ability to shorten development time and facilitate the production of functional Diagnostic Lateral Flow Cartridge models. Step 2: Analyze Capabilities and Hazards In previous medical design projects we had been able to machine prototypes that were more than adequate given product specifications. Although we had the capability of doing so in designing the Diagnostic Lateral Flow Cartridge, the hazard involved was that a machined prototype, although providing suitable accuracy would require too much time. In this application, tesNike Schuhe
The Marketplace Challenge: Two words best encompass a challenge often faced by medical device designers and manufacturers: "speed" and "reliability". Intense competitive pressures, combined with demands from the medical community for solutions to combat the spread of disease, have created a marketplace in which even months can be too long for companies to react and produce relevant products. And yet, speed-to-market must be achieved without compromising the critical need for medical products to perform as required in accordance with highly technical specifications, many of which are predicated upon accuracy and durability. Symbient Product Development strives to achieve both speed and reliability by blending current science and engineering theory with solid design principles for the creation of medical device and diagnostic products. In that process, our ability to evaluate a design and anticipate product performance is often highly dependent upon the quality and comprehensiveness of prototyping technology. This case study will focus on the vital role played by WaterShed?XC 11122, a rapid prototyping resin from DSM Somos, which helps to ensure a remarkably fast turnaround coupled with the ability to replicate actual performance attributes of production-grade engineered thermoplastics. The Product Challenge: Symbient Product Development was given the opportunity to participate in a fast-timeline project to develop a Diagnostic Lateral Flow Cartridge. This device uses a lateral flow test strip to evaluate, within a matter of minutes at the point-of-care, whether or not a patient is infected with a virus. In conducting such a test, a fluid sample is collected from the patient and mixed with a buffer, after which several drops of the combined sample and buffer are added to a sample well. The sample is applied, test strips run, and a clinician then places the Diagnostic Lateral Flow Cartridge into an instrument that reads the test result. Our design assignment, in parallel with the development of the internal cartridge features, was to develop an exterior industrial design that includes the user interface, features to aid in handling and insertion into the instrument, and aesthetic factors intended to give the product a unique appearance. In accepting this product design project Symbient Product Development committed to the necessity of completing our responsibilities within four months ?an extremely fast turnaround 梬ithout sacrificing the quality of prototyping evaluations that would assure the end-user that the Diagnostic Lateral Flow Cartridge would perform as required. Solving the Challenges: Our approach to meeting challenges of both the medical device marketplace and the individual product for which we were responsible, was based on a procedure almost identical to the accepted four-step plan for handling a medical emergency: Step 1: Establish a Planning Team Our first goal was to ensure that our vision and expertise aligned with our client's vision and expectations. This required input from our designers, engineers and administrators, but also the experience of professional resources such as DSM Somos, a world leader in high-performance stereolithography resins. We knew, from prior experience, that DSM's rapid prototyping materials would provide us with the ability to shorten development time and facilitate the production of functional Diagnostic Lateral Flow Cartridge models. Step 2: Analyze Capabilities and Hazards In previous medical design projects we had been able to machine prototypes that were more than adequate given product specifications. Although we had the capability of doing so in designing the Diagnostic Lateral Flow Cartridge, the hazard involved was that a machined prototype, although providing suitable accuracy would require too much time. In this application, tesNike SchuheSpeed and Reliability in Medical Device Development
Source: Release Date:2009-12-08 391
A case study featuring Symbient Product Development and DSM Somos  The Marketplace Challenge: Two words best encompass a challenge often faced by medical device designers and manufacturers: "speed" and "reliability". Intense competitive pressures, combined with demands from the medical community for solutions to combat the spread of disease, have created a marketplace in which even months can be too long for companies to react and produce relevant products. And yet, speed-to-market must be achieved without compromising the critical need for medical products to perform as required in accordance with highly technical specifications, many of which are predicated upon accuracy and durability. Symbient Product Development strives to achieve both speed and reliability by blending current science and engineering theory with solid design principles for the creation of medical device and diagnostic products. In that process, our ability to evaluate a design and anticipate product performance is often highly dependent upon the quality and comprehensiveness of prototyping technology. This case study will focus on the vital role played by WaterShed?XC 11122, a rapid prototyping resin from DSM Somos, which helps to ensure a remarkably fast turnaround coupled with the ability to replicate actual performance attributes of production-grade engineered thermoplastics. The Product Challenge: Symbient Product Development was given the opportunity to participate in a fast-timeline project to develop a Diagnostic Lateral Flow Cartridge. This device uses a lateral flow test strip to evaluate, within a matter of minutes at the point-of-care, whether or not a patient is infected with a virus. In conducting such a test, a fluid sample is collected from the patient and mixed with a buffer, after which several drops of the combined sample and buffer are added to a sample well. The sample is applied, test strips run, and a clinician then places the Diagnostic Lateral Flow Cartridge into an instrument that reads the test result. Our design assignment, in parallel with the development of the internal cartridge features, was to develop an exterior industrial design that includes the user interface, features to aid in handling and insertion into the instrument, and aesthetic factors intended to give the product a unique appearance. In accepting this product design project Symbient Product Development committed to the necessity of completing our responsibilities within four months ?an extremely fast turnaround 梬ithout sacrificing the quality of prototyping evaluations that would assure the end-user that the Diagnostic Lateral Flow Cartridge would perform as required. Solving the Challenges: Our approach to meeting challenges of both the medical device marketplace and the individual product for which we were responsible, was based on a procedure almost identical to the accepted four-step plan for handling a medical emergency: Step 1: Establish a Planning Team Our first goal was to ensure that our vision and expertise aligned with our client's vision and expectations. This required input from our designers, engineers and administrators, but also the experience of professional resources such as DSM Somos, a world leader in high-performance stereolithography resins. We knew, from prior experience, that DSM's rapid prototyping materials would provide us with the ability to shorten development time and facilitate the production of functional Diagnostic Lateral Flow Cartridge models. Step 2: Analyze Capabilities and Hazards In previous medical design projects we had been able to machine prototypes that were more than adequate given product specifications. Although we had the capability of doing so in designing the Diagnostic Lateral Flow Cartridge, the hazard involved was that a machined prototype, although providing suitable accuracy would require too much time. In this application, tesNike Schuhe
The Marketplace Challenge: Two words best encompass a challenge often faced by medical device designers and manufacturers: "speed" and "reliability". Intense competitive pressures, combined with demands from the medical community for solutions to combat the spread of disease, have created a marketplace in which even months can be too long for companies to react and produce relevant products. And yet, speed-to-market must be achieved without compromising the critical need for medical products to perform as required in accordance with highly technical specifications, many of which are predicated upon accuracy and durability. Symbient Product Development strives to achieve both speed and reliability by blending current science and engineering theory with solid design principles for the creation of medical device and diagnostic products. In that process, our ability to evaluate a design and anticipate product performance is often highly dependent upon the quality and comprehensiveness of prototyping technology. This case study will focus on the vital role played by WaterShed?XC 11122, a rapid prototyping resin from DSM Somos, which helps to ensure a remarkably fast turnaround coupled with the ability to replicate actual performance attributes of production-grade engineered thermoplastics. The Product Challenge: Symbient Product Development was given the opportunity to participate in a fast-timeline project to develop a Diagnostic Lateral Flow Cartridge. This device uses a lateral flow test strip to evaluate, within a matter of minutes at the point-of-care, whether or not a patient is infected with a virus. In conducting such a test, a fluid sample is collected from the patient and mixed with a buffer, after which several drops of the combined sample and buffer are added to a sample well. The sample is applied, test strips run, and a clinician then places the Diagnostic Lateral Flow Cartridge into an instrument that reads the test result. Our design assignment, in parallel with the development of the internal cartridge features, was to develop an exterior industrial design that includes the user interface, features to aid in handling and insertion into the instrument, and aesthetic factors intended to give the product a unique appearance. In accepting this product design project Symbient Product Development committed to the necessity of completing our responsibilities within four months ?an extremely fast turnaround 梬ithout sacrificing the quality of prototyping evaluations that would assure the end-user that the Diagnostic Lateral Flow Cartridge would perform as required. Solving the Challenges: Our approach to meeting challenges of both the medical device marketplace and the individual product for which we were responsible, was based on a procedure almost identical to the accepted four-step plan for handling a medical emergency: Step 1: Establish a Planning Team Our first goal was to ensure that our vision and expertise aligned with our client's vision and expectations. This required input from our designers, engineers and administrators, but also the experience of professional resources such as DSM Somos, a world leader in high-performance stereolithography resins. We knew, from prior experience, that DSM's rapid prototyping materials would provide us with the ability to shorten development time and facilitate the production of functional Diagnostic Lateral Flow Cartridge models. Step 2: Analyze Capabilities and Hazards In previous medical design projects we had been able to machine prototypes that were more than adequate given product specifications. Although we had the capability of doing so in designing the Diagnostic Lateral Flow Cartridge, the hazard involved was that a machined prototype, although providing suitable accuracy would require too much time. In this application, tesNike Schuhe
 The Marketplace Challenge: Two words best encompass a challenge often faced by medical device designers and manufacturers: "speed" and "reliability". Intense competitive pressures, combined with demands from the medical community for solutions to combat the spread of disease, have created a marketplace in which even months can be too long for companies to react and produce relevant products. And yet, speed-to-market must be achieved without compromising the critical need for medical products to perform as required in accordance with highly technical specifications, many of which are predicated upon accuracy and durability. Symbient Product Development strives to achieve both speed and reliability by blending current science and engineering theory with solid design principles for the creation of medical device and diagnostic products. In that process, our ability to evaluate a design and anticipate product performance is often highly dependent upon the quality and comprehensiveness of prototyping technology. This case study will focus on the vital role played by WaterShed?XC 11122, a rapid prototyping resin from DSM Somos, which helps to ensure a remarkably fast turnaround coupled with the ability to replicate actual performance attributes of production-grade engineered thermoplastics. The Product Challenge: Symbient Product Development was given the opportunity to participate in a fast-timeline project to develop a Diagnostic Lateral Flow Cartridge. This device uses a lateral flow test strip to evaluate, within a matter of minutes at the point-of-care, whether or not a patient is infected with a virus. In conducting such a test, a fluid sample is collected from the patient and mixed with a buffer, after which several drops of the combined sample and buffer are added to a sample well. The sample is applied, test strips run, and a clinician then places the Diagnostic Lateral Flow Cartridge into an instrument that reads the test result. Our design assignment, in parallel with the development of the internal cartridge features, was to develop an exterior industrial design that includes the user interface, features to aid in handling and insertion into the instrument, and aesthetic factors intended to give the product a unique appearance. In accepting this product design project Symbient Product Development committed to the necessity of completing our responsibilities within four months ?an extremely fast turnaround 梬ithout sacrificing the quality of prototyping evaluations that would assure the end-user that the Diagnostic Lateral Flow Cartridge would perform as required. Solving the Challenges: Our approach to meeting challenges of both the medical device marketplace and the individual product for which we were responsible, was based on a procedure almost identical to the accepted four-step plan for handling a medical emergency: Step 1: Establish a Planning Team Our first goal was to ensure that our vision and expertise aligned with our client's vision and expectations. This required input from our designers, engineers and administrators, but also the experience of professional resources such as DSM Somos, a world leader in high-performance stereolithography resins. We knew, from prior experience, that DSM's rapid prototyping materials would provide us with the ability to shorten development time and facilitate the production of functional Diagnostic Lateral Flow Cartridge models. Step 2: Analyze Capabilities and Hazards In previous medical design projects we had been able to machine prototypes that were more than adequate given product specifications. Although we had the capability of doing so in designing the Diagnostic Lateral Flow Cartridge, the hazard involved was that a machined prototype, although providing suitable accuracy would require too much time. In this application, tesNike Schuhe
The Marketplace Challenge: Two words best encompass a challenge often faced by medical device designers and manufacturers: "speed" and "reliability". Intense competitive pressures, combined with demands from the medical community for solutions to combat the spread of disease, have created a marketplace in which even months can be too long for companies to react and produce relevant products. And yet, speed-to-market must be achieved without compromising the critical need for medical products to perform as required in accordance with highly technical specifications, many of which are predicated upon accuracy and durability. Symbient Product Development strives to achieve both speed and reliability by blending current science and engineering theory with solid design principles for the creation of medical device and diagnostic products. In that process, our ability to evaluate a design and anticipate product performance is often highly dependent upon the quality and comprehensiveness of prototyping technology. This case study will focus on the vital role played by WaterShed?XC 11122, a rapid prototyping resin from DSM Somos, which helps to ensure a remarkably fast turnaround coupled with the ability to replicate actual performance attributes of production-grade engineered thermoplastics. The Product Challenge: Symbient Product Development was given the opportunity to participate in a fast-timeline project to develop a Diagnostic Lateral Flow Cartridge. This device uses a lateral flow test strip to evaluate, within a matter of minutes at the point-of-care, whether or not a patient is infected with a virus. In conducting such a test, a fluid sample is collected from the patient and mixed with a buffer, after which several drops of the combined sample and buffer are added to a sample well. The sample is applied, test strips run, and a clinician then places the Diagnostic Lateral Flow Cartridge into an instrument that reads the test result. Our design assignment, in parallel with the development of the internal cartridge features, was to develop an exterior industrial design that includes the user interface, features to aid in handling and insertion into the instrument, and aesthetic factors intended to give the product a unique appearance. In accepting this product design project Symbient Product Development committed to the necessity of completing our responsibilities within four months ?an extremely fast turnaround 梬ithout sacrificing the quality of prototyping evaluations that would assure the end-user that the Diagnostic Lateral Flow Cartridge would perform as required. Solving the Challenges: Our approach to meeting challenges of both the medical device marketplace and the individual product for which we were responsible, was based on a procedure almost identical to the accepted four-step plan for handling a medical emergency: Step 1: Establish a Planning Team Our first goal was to ensure that our vision and expertise aligned with our client's vision and expectations. This required input from our designers, engineers and administrators, but also the experience of professional resources such as DSM Somos, a world leader in high-performance stereolithography resins. We knew, from prior experience, that DSM's rapid prototyping materials would provide us with the ability to shorten development time and facilitate the production of functional Diagnostic Lateral Flow Cartridge models. Step 2: Analyze Capabilities and Hazards In previous medical design projects we had been able to machine prototypes that were more than adequate given product specifications. Although we had the capability of doing so in designing the Diagnostic Lateral Flow Cartridge, the hazard involved was that a machined prototype, although providing suitable accuracy would require too much time. In this application, tesNike Schuhe
You May Like

 iConnectHub
iConnectHub
 Login/Register
Login/Register Supplier Login
Supplier Login


























