THE U.S. Food and Drug Administration has approved the Maestro Rechargeable System for certain obese adults, the first weight loss treatment device that targets the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness.
The Maestro Rechargeable System is approved to treat patients aged 18 and older who have not been able to lose weight with a weight loss program, and who have a body mass index of 35 to 45 with at least one other obesity-related condition, such as type 2 diabetes. The surgical implant from EnteroMedics (St. Paul, Minnesota) is the first FDA-approved obesity device since 2007.
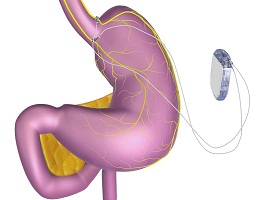 The Maestro System is implanted in a short outpatient procedure, which is completely reversible and does not require altering the patient’s anatomy, restricting the digestive system, or preventing absorption of nutrients (Photo: EnteroMedics)
The Maestro System is implanted in a short outpatient procedure, which is completely reversible and does not require altering the patient’s anatomy, restricting the digestive system, or preventing absorption of nutrients (Photo: EnteroMedics)
The pacemaker-like device that is implanted, usually in an outpatient procedure, to control both hunger and fullness by intermittently blocking the primary nerve which regulates the digestive system, the vagus nerve.
The Maestro consists of a rechargeable electrical pulse generator, wire leads and electrodes implanted surgically into the abdomen. It works by sending intermittent electrical pulses to the trunks in the abdominal vagus nerve, which is involved in regulating stomach emptying and signaling to the brain that the stomach feels empty or full. External controllers allow the patient to charge the device and allow health care professionals to adjust the device’s settings in order to provide optimal therapy with minimal side effects.
The FDA approval was based partly on results of a clinical trial that included 233 patients with a BMI of 35 or greater who had all received the implant. The experimental group of 157 patients received an activated Maestro device while the control group of 76 patients received a Maestro electrical pulse generator that was not activated.
Following a 12-month evaluation of weight loss and adverse events, the study found that the experimental group lost 8.5% more of its excess weight than the control group. About half (52.5%) of the patients in the experimental group lost at least 20% of their excess weight, and over a third (38.3%) of patients in the experimental group lost at least 25% of their excess weight.
Serious adverse events reported in the clinical study included nausea, pain at the neuroregulator site, vomiting as well as surgical complications. Other adverse events included pain, heartburn, problems swallowing, belching, mild nausea and chest pain.
As part of the approval, EnteroMedics must conduct a five-year post approval study that will follow at least 100 patients and collect additional safety and effectiveness data, including weight loss, adverse events, surgical revisions and explants and changes in obesity-related conditions.
Nike Zoom Kobe Shoes
 iConnectHub
iConnectHub
 Login/Register
Login/Register Supplier Login
Supplier Login



























