WARNING letters sent by U.S. Food and Drug Administration (FDA) to companies seek to clarify the differences between regulated drugs and medical devices, on the one hand, and cosmeceuticals and cosmetic devices, on the other.
The letters were sent in January to Chaga Mountain, Inc. and DermaPen LLC following inspections conducted at the companies’ facilities and a review of the claims made for their respective products in 2014.
 The FDA warned Chaga Mountain that it found serious violations of the Federal Food, Drug, & Cosmetic Act (FD&C Act) and its implementing regulations, particularly marketing cosmetic products as drugs. Through the review of the firm’s website in November 2014, the agency said Chaga Skin Cream and Lip Balm, Chaga Mushroom Tincture/Extract Alcohol Free, and two tea products were being sold with natural ingredients including natural essential oils which the firm’s website promotes for conditions that cause the products to be drugs. The warning also mentioned the used of adulterated dietary supplements and misbranded dietary supplements.
The FDA warned Chaga Mountain that it found serious violations of the Federal Food, Drug, & Cosmetic Act (FD&C Act) and its implementing regulations, particularly marketing cosmetic products as drugs. Through the review of the firm’s website in November 2014, the agency said Chaga Skin Cream and Lip Balm, Chaga Mushroom Tincture/Extract Alcohol Free, and two tea products were being sold with natural ingredients including natural essential oils which the firm’s website promotes for conditions that cause the products to be drugs. The warning also mentioned the used of adulterated dietary supplements and misbranded dietary supplements.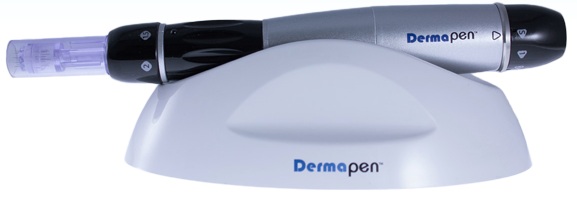 In its letter to DermaPen, the FDA noted the company’s Derma Pen Auto-Microneedle Therapy System requires premarket approval (PMA) or an approved application for an investigational device exemption (IDE). Although the device is designed to improve a person's appearance, claims made in brochures for the DermaPen device to diagnose or treat a medical condition or affect the structure or function of the body makes them medical devices under the FD&C Act, the agency also said.
In its letter to DermaPen, the FDA noted the company’s Derma Pen Auto-Microneedle Therapy System requires premarket approval (PMA) or an approved application for an investigational device exemption (IDE). Although the device is designed to improve a person's appearance, claims made in brochures for the DermaPen device to diagnose or treat a medical condition or affect the structure or function of the body makes them medical devices under the FD&C Act, the agency also said.
The FD&C Act requires medical device manufacturers to obtain marketing clearance for their products before offering them for sale [FD&C Act, section 501(f)(1)]. The law does not require clearance or approval to market cosmetic products or ingredients, with the exception of color additives. In addition, medical devices are subject to the Quality System Regulation (21 CFR part 820). Cosmetics are not subject to this regulation.
Zoom Lebron XV 15 Low
 iConnectHub
iConnectHub
 Login/Register
Login/Register Supplier Login
Supplier Login



























